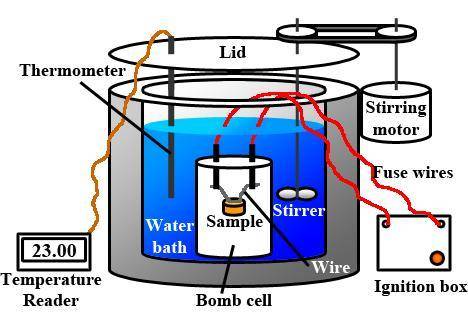
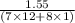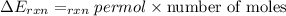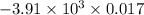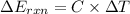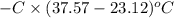When 1.550 g of liquid hexane (c6h14) undergoes combustion in a bomb calorimeter, the temperature rises from 25.87∘c to 38.13∘c. find δerxn for the reaction in kj/mol hexane. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.73 kj/∘c. express your answer in kilojoules per mole to three significant figures.2. the combustion of toluene has a δerxn of –3.91×103 kj/mol. when 1.55 g of toluene (c7h8) undergoes combustion in a bomb calorimeter, the temperature rises from 23.12∘c to 37.57∘c. find the heat capacity of the bomb calorimeter. express the heat capacity in kilojoules per degree celsius to three significant

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
When 1.550 g of liquid hexane (c6h14) undergoes combustion in a bomb calorimeter, the temperature ri...
Questions



Mathematics, 30.04.2021 17:30

Health, 30.04.2021 17:30






English, 30.04.2021 17:30

Mathematics, 30.04.2021 17:30

Mathematics, 30.04.2021 17:30


Mathematics, 30.04.2021 17:30

Chemistry, 30.04.2021 17:30



Biology, 30.04.2021 17:30


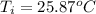 ,
, 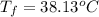
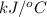
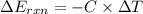
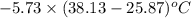
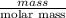
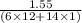
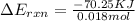
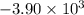 kJ/mol
kJ/mol
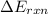 for the reaction in kJ/mol hexane is
for the reaction in kJ/mol hexane is