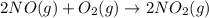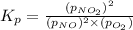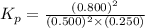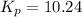Chemistry, 04.12.2019 01:31 Kingzion5775
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 bar for all species. for the reaction
2 no ( g ) + o 2 ( g ) > 2 no 2 ( g )
the standard change in gibbs free energy is δ g ° = − 32.8 kj / mol . what is δ g for this reaction at 298 k when the partial pressures are:
pno = 0.500 bar , po2 = 0.250 bar , and pno 2 = 0.800 bar
deltag = ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 bar for all specie...
Questions

English, 08.02.2021 22:00

History, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00




Mathematics, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00






English, 08.02.2021 22:00

Arts, 08.02.2021 22:00

Health, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00


Arts, 08.02.2021 22:00

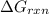 is, -27.0kJ/mole
is, -27.0kJ/mole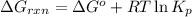 ............(1)
............(1)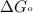 = standard Gibbs free energy = -32.8 kJ
= standard Gibbs free energy = -32.8 kJ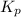 = equilibrium constant
= equilibrium constant