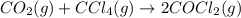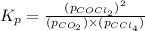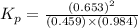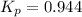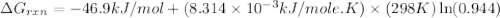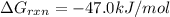Chemistry, 04.12.2019 00:31 jonquil201
Consider the reaction: co2(g) + ccl4(g) ⇌ 2 cocl2(g) δg° = 46.9 kj under the following conditions at 25 oc: latex: p_{co_2}p c o 2= 0.459 atm, latex: p_{ccl_4}p c c l 4= 0.984 atm, and latex: p_{cocl_2}p c o c l 2= 0.653 atm, δg for the reaction is , and the forward the reaction is

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1


You know the right answer?
Consider the reaction: co2(g) + ccl4(g) ⇌ 2 cocl2(g) δg° = 46.9 kj under the following conditions a...
Questions


History, 06.07.2019 11:30




Mathematics, 06.07.2019 11:30


Mathematics, 06.07.2019 11:30


History, 06.07.2019 11:30

History, 06.07.2019 11:30




English, 06.07.2019 11:30

English, 06.07.2019 11:30

Biology, 06.07.2019 11:30

Mathematics, 06.07.2019 11:30


Mathematics, 06.07.2019 11:30

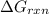 is,
is, 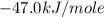
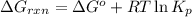 ............(1)
............(1)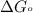 = standard Gibbs free energy = 46.9 kJ
= standard Gibbs free energy = 46.9 kJ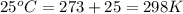
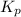 = equilibrium constant
= equilibrium constant