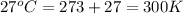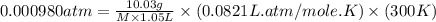Chemistry, 04.12.2019 00:31 zuleidysnegron
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at 27°c. the solution's osmotic pressure at 27°c is found to be 0.745 torr. calculate the molar mass of g/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at...
Questions


Physics, 24.06.2019 19:00

Biology, 24.06.2019 19:00

History, 24.06.2019 19:00

English, 24.06.2019 19:00

English, 24.06.2019 19:00

Mathematics, 24.06.2019 19:00


English, 24.06.2019 19:00

Mathematics, 24.06.2019 19:00

History, 24.06.2019 19:00

Mathematics, 24.06.2019 19:00


Mathematics, 24.06.2019 19:00

English, 24.06.2019 19:00

History, 24.06.2019 19:00




Mathematics, 24.06.2019 19:00

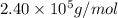
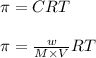
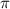 = osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)
= osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)