
Chemistry, 03.12.2019 22:31 ronnie7898
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0 ml of 1 m h2so4. a 25.00 ml aliquot is analyzed by adding ki and titrating the i3^- that forms with s2o3^2-. the end point was reached following the addition of 13.02 ml of 0.03247 m na2s2o3. calculate the weight percent of ce^4+ in the sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0 ml of 1 m h2so4....
Questions


Mathematics, 01.12.2020 19:50

Mathematics, 01.12.2020 19:50


Biology, 01.12.2020 19:50

English, 01.12.2020 19:50

History, 01.12.2020 19:50



History, 01.12.2020 19:50

Physics, 01.12.2020 19:50




Mathematics, 01.12.2020 19:50

Mathematics, 01.12.2020 19:50



Mathematics, 01.12.2020 19:50

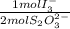 = 2,114x10⁻⁴ moles of I₃⁻
= 2,114x10⁻⁴ moles of I₃⁻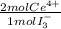 = 4,228x10⁻⁴ moles of Ce(IV).
= 4,228x10⁻⁴ moles of Ce(IV). 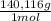 = 0,05924 g of Ce(IV)
= 0,05924 g of Ce(IV)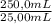 =0,5924g of Ce(IV) in the sample
=0,5924g of Ce(IV) in the sample

