
Chemistry, 03.12.2019 22:31 allieballey0727
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2 o ( l ) δ h ∘ rxn = − 571.6 kj calculate the value of δ h ∘ rxn for 2 f 2 ( g ) + 2 h 2 o ( l ) ⟶ 4 hf ( g ) + o 2 ( g )

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

You know the right answer?
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2...
Questions

Mathematics, 30.10.2020 20:20

Mathematics, 30.10.2020 20:20

Social Studies, 30.10.2020 20:20






History, 30.10.2020 20:20


Mathematics, 30.10.2020 20:20



Mathematics, 30.10.2020 20:20



Mathematics, 30.10.2020 20:20



Mathematics, 30.10.2020 20:20

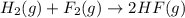
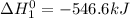 (1)
(1)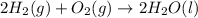
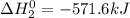 (2)
(2)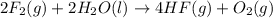
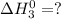 (3)
(3)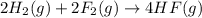
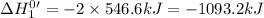 (1')
(1')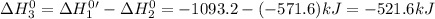 .
.

