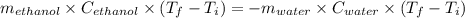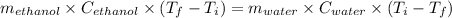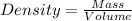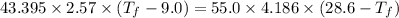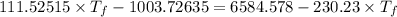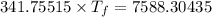Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
If 55.0 ml of ethanol (density=0.789g/ml)) initially at 9.0 ∘c is mixed with 55.0 ml of water (densi...
Questions

Spanish, 13.05.2021 07:20


Mathematics, 13.05.2021 07:20

Mathematics, 13.05.2021 07:20



Mathematics, 13.05.2021 07:20

English, 13.05.2021 07:20

Chemistry, 13.05.2021 07:20

Mathematics, 13.05.2021 07:20

Chemistry, 13.05.2021 07:20


History, 13.05.2021 07:20

Physics, 13.05.2021 07:20



Mathematics, 13.05.2021 07:20

Mathematics, 13.05.2021 07:20

History, 13.05.2021 07:20

Physics, 13.05.2021 07:20