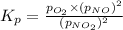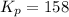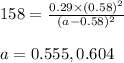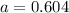Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1


Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
At 1000 k, a sample of pure no2 gas decomposes. 2 no2(g) equilibrium reaction arrow 2 no(g) + o2(g)...
Questions

Health, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

English, 20.10.2020 21:01



English, 20.10.2020 21:01



Mathematics, 20.10.2020 21:01



Business, 20.10.2020 21:01

English, 20.10.2020 21:01



Spanish, 20.10.2020 21:01


Biology, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

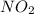 in the mixture is 0.58 atm and 0.024 atm respectively.
in the mixture is 0.58 atm and 0.024 atm respectively.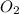 = 0.29 atm
= 0.29 atm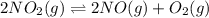
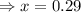
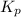 for above equation follows:
for above equation follows: