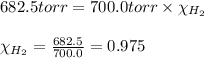Chemistry, 03.12.2019 18:31 taridunkley724
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of 700.0 torr. the partial pressure of water vapor at 20.00∘c is 17.5 torr. calculate the mole fraction of h2 gas in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of...
Questions

Mathematics, 11.02.2021 14:00

Biology, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

English, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00


History, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

English, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

History, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00



History, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00


World Languages, 11.02.2021 14:00

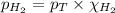
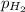 = partial pressure of hydrogen gas = 682.5 torr
= partial pressure of hydrogen gas = 682.5 torr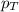 = total pressure = 700.0 torr
= total pressure = 700.0 torr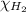 = mole fraction of hydrogen gas = ?
= mole fraction of hydrogen gas = ?