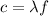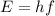Chemistry, 03.12.2019 06:31 genyjoannerubiera
Fireworks that contain metallic salts such as sodium, strontium, and barium can generate bright colors. a technician investigates what colors are produced by the metallic salts by performing flame tests. during a flame test, a metallic salt is heated in the flame of a gas burner. each metallic salt emits a characteristic colored light in the flame. 3. state how bright-line spectra viewed through a spectroscope can be used to identify the metal ions in the salts used in the flame tests. 4. explain, in terms of electrons, how a strontium salt emits colored light. 5. explain why the electron configuration of 2-7-1-1 represents a sodium atom in an excited state.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Fireworks that contain metallic salts such as sodium, strontium, and barium can generate bright colo...
Questions

Biology, 16.01.2020 13:31


English, 16.01.2020 13:31

History, 16.01.2020 13:31

Biology, 16.01.2020 13:31

Social Studies, 16.01.2020 13:31

History, 16.01.2020 13:31




Chemistry, 16.01.2020 13:31

Mathematics, 16.01.2020 13:31


History, 16.01.2020 13:31




Arts, 16.01.2020 13:31


Mathematics, 16.01.2020 13:31