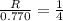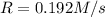Chemistry, 03.12.2019 05:31 caldonia2018
At a certain concentration of n2 and o3, the initial rate of reaction is 0.770 m / s. what would the initial rate of the reaction be if the concentration of n2 were halved? be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2

Chemistry, 23.06.2019 14:40
Uuestons niuthe no. of millimoles of hcl required to neutralize 10 ml of 0.2 m na2co3 is(a) 2.0 m mole(b) 4.0 m mole(c) 0.2 m mole(d) 0.4 m mole
Answers: 1

You know the right answer?
At a certain concentration of n2 and o3, the initial rate of reaction is 0.770 m / s. what would the...
Questions

Mathematics, 22.04.2021 14:00

Social Studies, 22.04.2021 14:00

Physics, 22.04.2021 14:00



English, 22.04.2021 14:00





English, 22.04.2021 14:00


History, 22.04.2021 14:00

Biology, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00



Mathematics, 22.04.2021 14:00


Mathematics, 22.04.2021 14:00

![rate=k[N_2]^2[H_2]^2](/tpl/images/0400/5502/ac047.png)
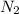 and
and 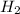 , the initial rate of reaction is 0.770 M/s. What would the initial rate of the reaction be if the concentration of
, the initial rate of reaction is 0.770 M/s. What would the initial rate of the reaction be if the concentration of ![0.770=k[N_2]^2[H_2]^2](/tpl/images/0400/5502/83364.png) ....(1)
....(1)![R=k(\frac{[N_2]}{2})^2[H_2]^2](/tpl/images/0400/5502/0c6c2.png) ....(2)
....(2)![\frac{R}{0.770}=\frac{k(\frac{[N_2]}{2})^2[H_2]^2}{k[N_2]^2[H_2]^2}](/tpl/images/0400/5502/4ab48.png)