
Two flasks of equal volume and at the same temperature contain different gases. one flask contains 5.0 g of o2, and the other flask contains 5.0 g of h2. is each of the following statements true or false? explain.
a) true. because the gases have the same volumes, they must have the same number of molecules.
b) false. because the molar mass of o2 is greater than the molar mass of h2, 5.0g of o2 will contain fewer molecules than 5.0 g of h2.
c)false. depending on the pressure each flask may contain different numbers of molecules.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Two flasks of equal volume and at the same temperature contain different gases. one flask contains 5...
Questions

Mathematics, 28.01.2020 17:02



Mathematics, 28.01.2020 17:02

Mathematics, 28.01.2020 17:02


Advanced Placement (AP), 28.01.2020 17:02


English, 28.01.2020 17:02


Biology, 28.01.2020 17:03

Mathematics, 28.01.2020 17:03






Mathematics, 28.01.2020 17:03


Mathematics, 28.01.2020 17:03

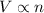
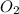 =
= 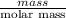
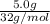
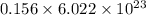
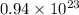 molecules
molecules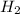 =
= 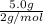
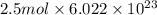
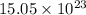 molecules
molecules![O_{2} will contain same molecules as 5.0 g of [tex]H_{2}](/tpl/images/0400/5815/5c250.png) is not true.
is not true.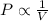 (at constant temperature and number of moles)
(at constant temperature and number of moles)

