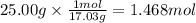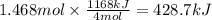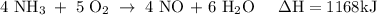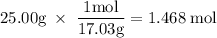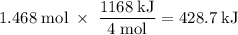Chemistry, 03.12.2019 04:31 averylivinglife2041
How much heat is absorbed/released when 25.00 g of nh3(g) reacts in the presence of excess o2(g) to produce no(g) and h2o(l) according to the following chemical equation? 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(l) δh° = 1168 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
How much heat is absorbed/released when 25.00 g of nh3(g) reacts in the presence of excess o2(g) to...
Questions


Mathematics, 12.12.2019 00:31




Biology, 12.12.2019 00:31


Mathematics, 12.12.2019 00:31

Biology, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31


History, 12.12.2019 00:31


Mathematics, 12.12.2019 00:31



Business, 12.12.2019 00:31


Mathematics, 12.12.2019 00:31