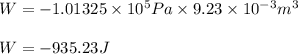Chemistry, 03.12.2019 04:31 jasmine2919
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heated, thus inflating the bag. 2 nan 3 ( s ) ⟶ 2 na ( s ) + 3 n 2 ( g ) calculate the value of work, w , for the system if 16.5 g nan 3 reacts completely at 1.00 atm and 22 ∘ c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3


Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heat...
Questions







Mathematics, 27.06.2019 10:30

History, 27.06.2019 10:30


English, 27.06.2019 10:30


Health, 27.06.2019 10:30








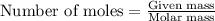
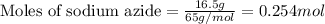
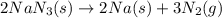
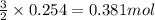 of nitrogen gas
of nitrogen gas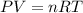
![22^oC=[22+273]K=295K](/tpl/images/0400/4731/7919f.png)
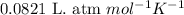
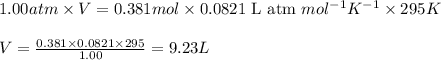
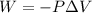
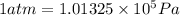 (Conversion factor: 1 atm = 101325 Pa)
(Conversion factor: 1 atm = 101325 Pa)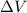 = change in volume =
= change in volume = 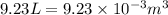 (Conversion factor:
(Conversion factor: 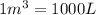 )
)