

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
What is the value for the reaction: n2(g) + 2 o2(g) --> n2o4(g) in terms of k values from the r...
Questions


Mathematics, 04.02.2020 19:51



Mathematics, 04.02.2020 19:51

English, 04.02.2020 19:51


History, 04.02.2020 19:51

Social Studies, 04.02.2020 19:51


Advanced Placement (AP), 04.02.2020 19:51

Mathematics, 04.02.2020 19:52



English, 04.02.2020 19:52

Mathematics, 04.02.2020 19:52



Mathematics, 04.02.2020 19:52

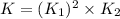
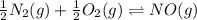
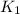
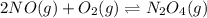
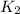
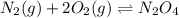
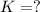
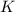 for the final reaction.
for the final reaction.

