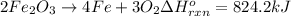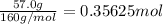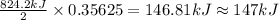Chemistry, 03.12.2019 03:31 dashavasilisk
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe + 3o2 deltah degree rxn = + 824.2 kj decomposition of 57.0 g of fe2o3 results in the release of 294 kj of heat. a. the absorption of 23500 kj of heat. b. the absorption of 147 kj of heat. c. the absorption of 294 kj of heat. d. the release of 23500 kj of heat. e. the release of 147 kj of heat.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

You know the right answer?
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe +...
Questions

Social Studies, 18.10.2019 10:20

Biology, 18.10.2019 10:20

Mathematics, 18.10.2019 10:20



Mathematics, 18.10.2019 10:20

Mathematics, 18.10.2019 10:20

Computers and Technology, 18.10.2019 10:20

Mathematics, 18.10.2019 10:20



Mathematics, 18.10.2019 10:20

Biology, 18.10.2019 10:20



Mathematics, 18.10.2019 10:20

Spanish, 18.10.2019 10:20