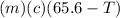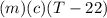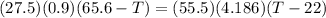Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
A27.5 −g aluminum block is warmed to 65.6 ∘c and plunged into an insulated beaker containing 55.5 g...
Questions

Social Studies, 23.08.2019 12:00

Chemistry, 23.08.2019 12:00

Arts, 23.08.2019 12:00


Mathematics, 23.08.2019 12:00

Mathematics, 23.08.2019 12:00



Biology, 23.08.2019 12:00

Computers and Technology, 23.08.2019 12:00

History, 23.08.2019 12:00

Mathematics, 23.08.2019 12:00

Mathematics, 23.08.2019 12:00






Chemistry, 23.08.2019 12:00