

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Given thath2(g) + f2(g) -> 2hf(g) => ∆h = -546.6 kj . mol-12h2(g) + o2(g) -> 2h20(l) =&g...
Questions




Mathematics, 21.01.2020 11:31

History, 21.01.2020 11:31


Biology, 21.01.2020 11:31

History, 21.01.2020 11:31

Computers and Technology, 21.01.2020 11:31


Mathematics, 21.01.2020 11:31


Geography, 21.01.2020 11:31


Health, 21.01.2020 11:31

History, 21.01.2020 11:31



Mathematics, 21.01.2020 11:31

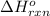 for the reaction is -521.6 kJ.
for the reaction is -521.6 kJ.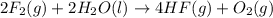
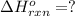
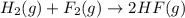
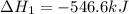 ( × 2)
( × 2)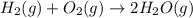
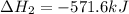
![\Delta H^o_{rxn}=[2\times \Delta H_1]+[1\times (-\Delta H_2)]](/tpl/images/0400/0579/648b7.png)
![\Delta H^o_{rxn}=[(2\times (-546.6))+(1\times (571.6))]=-521.6kJ](/tpl/images/0400/0579/83f77.png)


