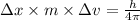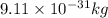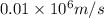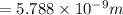Chemistry, 03.12.2019 00:31 Sariyahhall1
German physicist werner heisenberg related the uncertainty of an object's position ( δ x ) (δx) to the uncertainty in its velocity ( δ v ) (δv) δ x ≥ h 4 π m δ v δx≥h4πmδv where h h is planck's constant and m m is the mass of the object. the mass of an electron is 9.11 × 10 − 31 kg. 9.11×10−31 kg. what is the uncertainty in the position of an electron moving at 6.00 × 10 6 m/s 6.00×106 m/s with an uncertainty of δ v = 0.01 × 10 6 m/s ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
German physicist werner heisenberg related the uncertainty of an object's position ( δ x ) (δx) to t...
Questions


Health, 18.03.2020 00:22


Health, 18.03.2020 00:22


Social Studies, 18.03.2020 00:22


Biology, 18.03.2020 00:22

Mathematics, 18.03.2020 00:22

Mathematics, 18.03.2020 00:22

Computers and Technology, 18.03.2020 00:22

English, 18.03.2020 00:22

Mathematics, 18.03.2020 00:22



Chemistry, 18.03.2020 00:23


Mathematics, 18.03.2020 00:23

History, 18.03.2020 00:23

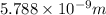 is the uncertainty in the position of a moving electron.
is the uncertainty in the position of a moving electron.