
Chemistry, 02.12.2019 23:31 tmrodriguez1
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of propane, and the standard enthalpy of combustion of gaseous propylene (c3h6) is –2058.3 kj per mole of propylene.
what is the standard enthalpy change for the following reaction at 25°c? c3h6(g) + h2(g) → c3h8(g)substance∆h°f (kj/mol)co2(g)–393.5h2o(l)–285.8

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1
You know the right answer?
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of pro...
Questions

History, 28.09.2019 12:30



History, 28.09.2019 12:30


Chemistry, 28.09.2019 12:30



Biology, 28.09.2019 12:30




Biology, 28.09.2019 12:30


Mathematics, 28.09.2019 12:30

History, 28.09.2019 12:30

History, 28.09.2019 12:30

Mathematics, 28.09.2019 12:30

Mathematics, 28.09.2019 12:30

Geography, 28.09.2019 12:30

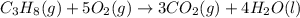
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0399/9365/6eb42.png)
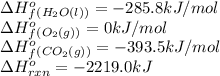
![-2219.0=[(3\times (-393.5))+(4\times (-285.8))]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times (0))]\\\\\Delta H^o_f_{(C_3H_8(g))}=-140.7kJ/mol](/tpl/images/0399/9365/987c0.png)
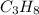 is -140.7 kJ/mol
is -140.7 kJ/mol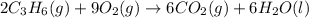
![\Delta H^o_{rxn}=[(6\times \Delta H^o_f_{(CO_2(g))})+(6\times \Delta H^o_f_{(H_2O(g))})]-[(2\times \Delta H^o_f_{(C_3H_6(g))})+(9\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0399/9365/b6201.png)
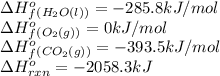
![-2058.3=[(6\times (-393.5))+(6\times (-285.8))]-[(2\times \Delta H^o_f_{(C_3H_6(g))})+(9\times (0))]\\\\\Delta H^o_f_{(C_3H_6(g))}=-1008.75kJ/mol](/tpl/images/0399/9365/ecedb.png)
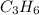 is -1008.75 kJ/mol
is -1008.75 kJ/mol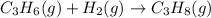
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(C_3H_8(g))})]-[(1\times \Delta H^o_f_{(C_3H_6(g))})+(1\times \Delta H^o_f_{(H_2(g))})]](/tpl/images/0399/9365/9b762.png)
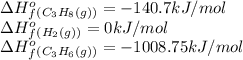
![\Delta H^o_{rxn}=[(1\times (-140.7))]-[(1\times (-1008.75))+(1\times (0))]\\\\\Delta H^o_{rxn}=868.05kJ](/tpl/images/0399/9365/ff366.png)


