
Chemistry, 02.12.2019 21:31 krystalhurst97
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change for the following reaction.
(note: show the math clearly and provide units in your set up) ( hf values in kj/mol are as follows: no2 32, h2o 286, hno3 207, no 90.)
3no2(g) h2o(l) 2hno3(aq) no(g) g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change...
Questions

English, 14.09.2020 23:01

French, 14.09.2020 23:01

English, 14.09.2020 23:01

Mathematics, 14.09.2020 23:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Physics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Health, 15.09.2020 01:01

History, 15.09.2020 01:01

Arts, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Biology, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Physics, 15.09.2020 01:01

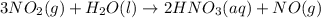
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0399/7869/76c37.png)
![\Delta H=[(n_{HNO_3}\times \Delta H_{HNO_3})+(n_{NO}\times \Delta H_{NO})]-[(n_{H_2O}\times \Delta H_{H_2O})+(n_{NO_2}\times \Delta H_{NO_2})]](/tpl/images/0399/7869/7081c.png)
![\Delta H=[(2\times -207)+(1\times 90)]-[(1\times -286)+(3\times 32)]](/tpl/images/0399/7869/1d6ad.png)



