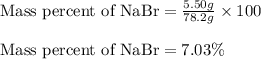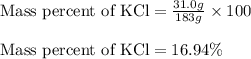Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Calculate the percent by mass of the solute in each of the following aqueous solutions: (a) 5.50 g...
Questions

Mathematics, 05.02.2020 04:02


Mathematics, 05.02.2020 04:02

Biology, 05.02.2020 04:02

Mathematics, 05.02.2020 04:02

Mathematics, 05.02.2020 04:02

English, 05.02.2020 04:02

Mathematics, 05.02.2020 04:02


Computers and Technology, 05.02.2020 04:02


Physics, 05.02.2020 04:02

Health, 05.02.2020 04:02

Mathematics, 05.02.2020 04:02



History, 05.02.2020 04:02


Mathematics, 05.02.2020 04:02

Mathematics, 05.02.2020 04:02

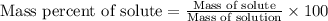 .......(1)
.......(1)