
Chemistry, 02.12.2019 19:31 elitehairnerd1964
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate forms and ag+ becomes negligibly small. which
of the following is the correct listing of the ions remaining in solution
in order of increasing concentration?
(a) po43- < no3- < na+
(b) po43- < na+ < no3-
(c) no3- < po43- < na+
(d) na+ < no3- < po43-
(e) na+ < po43- < no3-

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate fo...
a yellow precipitate fo...
Questions






Mathematics, 24.03.2021 05:10


Mathematics, 24.03.2021 05:10




Mathematics, 24.03.2021 05:10

Mathematics, 24.03.2021 05:10

Mathematics, 24.03.2021 05:10

Chemistry, 24.03.2021 05:10

Mathematics, 24.03.2021 05:10

Social Studies, 24.03.2021 05:10

Chemistry, 24.03.2021 05:10

Mathematics, 24.03.2021 05:10

Mathematics, 24.03.2021 05:10

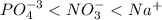
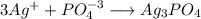
![[Ag^+]=0 M](/tpl/images/0399/5580/6ee64.png)
![[PO_4^{-3}]=0.5 M-0.5 M \frac{1 mol PO4}{3 mol Ag}=0.33 M](/tpl/images/0399/5580/8a590.png)
![[Na^+]=0.5 M * \frac{3 mol Na}{mol Na_3PO_4}=1.5 M](/tpl/images/0399/5580/07524.png)
![[NO_3^-]=0.5 M](/tpl/images/0399/5580/9ee3a.png)


