Chemistry, 02.12.2019 18:31 hayesvolcano
Write the half-reactions as they occur at each electrode and the net cell reaction for this electrochemical cell containing copper and silver. cu ( s ) ∣ ∣ cu 2 + ( aq ) ∥ ∥ ag + ( aq ) ∣ ∣ ag ( s ) anode: cathode: net cell reaction:

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Write the half-reactions as they occur at each electrode and the net cell reaction for this electroc...
Questions



Biology, 30.09.2019 16:00



History, 30.09.2019 16:00


Mathematics, 30.09.2019 16:00

Arts, 30.09.2019 16:00

Mathematics, 30.09.2019 16:00

Arts, 30.09.2019 16:00



Mathematics, 30.09.2019 16:00





Mathematics, 30.09.2019 16:00

Mathematics, 30.09.2019 16:00

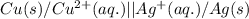
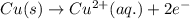
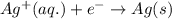 ( × 2)
( × 2)