31) the mass of an electron is approximately 1/1836
times the mass of
a) 1/1h
b) 2...

Chemistry, 02.12.2019 02:31 thaliavernazaa
31) the mass of an electron is approximately 1/1836
times the mass of
a) 1/1h
b) 2/1h
c) 3/1h
d) 4/2he


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Questions



History, 20.10.2019 12:20

Mathematics, 20.10.2019 12:20

History, 20.10.2019 12:20


English, 20.10.2019 12:20


English, 20.10.2019 12:20



Health, 20.10.2019 12:20





Health, 20.10.2019 12:20



English, 20.10.2019 12:20

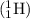 is the correct choice that is option A.
is the correct choice that is option A. times the mass of a proton, but an equal and opposite negative charge.
times the mass of a proton, but an equal and opposite negative charge.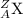 is any element represented here.
is any element represented here. is the number of protons as well as its atomic number.
is the number of protons as well as its atomic number.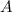 is the mass number which is the number of protons and neutrons in any atom.
is the mass number which is the number of protons and neutrons in any atom.

