
Chemistry, 01.12.2019 00:31 hei40563273
8. select the lattice energy for rubidium chloride from the following data (in kj/mol]
rb(s) > rb(g) = 85.8
ie1(rb) = 397.5
be(cl2) = 226
deltahf(rbcl) = -431
electron affinity cl = -332
a. -53.7
b. +53.7
c. -695
d. -808
e. +808

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
8. select the lattice energy for rubidium chloride from the following data (in kj/mol]
rb(s) &g...
rb(s) &g...
Questions



Geography, 17.11.2020 14:00


Mathematics, 17.11.2020 14:00

English, 17.11.2020 14:00



Engineering, 17.11.2020 14:00

Social Studies, 17.11.2020 14:00


Mathematics, 17.11.2020 14:00


Mathematics, 17.11.2020 14:00





Social Studies, 17.11.2020 14:00

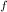 = -431 kJ/mol
= -431 kJ/mol![8. select the lattice energy for rubidium chloride from the following data (in kj/mol]rb(s) > rb](/tpl/images/0397/7697/0aca5.jpg)


