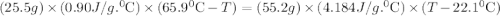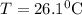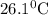Chemistry, 30.11.2019 07:31 Sylnatius6736
A25.5 −g aluminum block is warmed to 65.9 ∘c and plunged into an insulated beaker containing 55.2 g water initially at 22.1 ∘c. the aluminum and the water are allowed to come to thermal equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
A25.5 −g aluminum block is warmed to 65.9 ∘c and plunged into an insulated beaker containing 55.2 g...
Questions

Mathematics, 03.08.2019 23:50




Mathematics, 03.08.2019 23:50

Mathematics, 03.08.2019 23:50

English, 03.08.2019 23:50


English, 03.08.2019 23:50

Chemistry, 03.08.2019 23:50


Mathematics, 03.08.2019 23:50


Mathematics, 03.08.2019 23:50


Biology, 03.08.2019 23:50




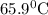 and plunged into an insulated beaker containing 55.2 g water initially at 22.1^{0}\textrm{C}. The aluminum and the water are allowed to come to thermal equilibrium. Assuming that no heat is lost, what is the final temperature of the water and aluminum?"
and plunged into an insulated beaker containing 55.2 g water initially at 22.1^{0}\textrm{C}. The aluminum and the water are allowed to come to thermal equilibrium. Assuming that no heat is lost, what is the final temperature of the water and aluminum?"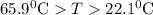
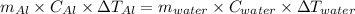
 represents change in temperature
represents change in temperature