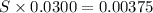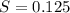What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.300 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions



Biology, 02.03.2021 19:30


Mathematics, 02.03.2021 19:30


Mathematics, 02.03.2021 19:30




Mathematics, 02.03.2021 19:30

World Languages, 02.03.2021 19:30

Arts, 02.03.2021 19:30

Mathematics, 02.03.2021 19:30

Mathematics, 02.03.2021 19:30



Chemistry, 02.03.2021 19:30

Mathematics, 02.03.2021 19:30

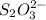 completely react with 1 mol of
completely react with 1 mol of 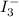 .
.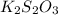 solution =
solution = 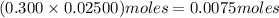
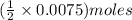 of
of 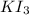 solution is S (M) then-
solution is S (M) then-