
Chemistry, 30.11.2019 05:31 rubyhart522
Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, including bottled water. a solution is made by adding 6.50 g of cacl2 to 60.0 ml of water at 25∘c. the density of the solvent at that temperature is 0.997 g/ml. calculate the mole percent of cacl2 in the solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, in...
Questions








Mathematics, 21.09.2019 04:30

Mathematics, 21.09.2019 04:30


Mathematics, 21.09.2019 04:30


Biology, 21.09.2019 04:30




Computers and Technology, 21.09.2019 04:30

Mathematics, 21.09.2019 04:30


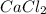 in solution is 1.71%
in solution is 1.71%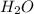 = 18.02 g/mol
= 18.02 g/mol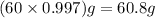
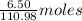 of
of 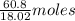 of
of 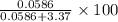 % = 1.71%
% = 1.71%


