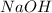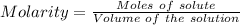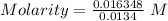Chemistry, 30.11.2019 05:31 brockandersin
The dissolution of 0.200 l of sulfur dioxide at 19 °c and 745 mmhg in water yields 500.0 ml of aqueous sulfurous acid. the solution is titrated with 13.4 ml of sodium hydroxide. what is the molarity of naoh?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
The dissolution of 0.200 l of sulfur dioxide at 19 °c and 745 mmhg in water yields 500.0 ml of aqueo...
Questions

Biology, 21.11.2019 05:31


Arts, 21.11.2019 05:31

Computers and Technology, 21.11.2019 05:31



Mathematics, 21.11.2019 05:31

Geography, 21.11.2019 05:31

Mathematics, 21.11.2019 05:31


History, 21.11.2019 05:31



Mathematics, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31



Physics, 21.11.2019 05:31

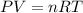
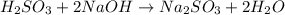
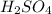 react with 2 moles of
react with 2 moles of