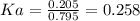Chemistry, 30.11.2019 05:31 lefthandeddolan
An acid-base indicator, hln, dissociates according to the following reaction in an aqueous solution. hinlag) in (aq) h (aq) the protonated form of the indicator, hln, has a molar absorptivity of 2929 m cm 1 and the deprotonated form, in has a molar absorptivity of 20060 m-1. cm 1 at 440 nm. the ph of a solution containing a mixture of hin and in s adjusted to 6.12. the total concentration of hin and in s 0.000127 m. the absorbance of this solution was measured at 440 nm in a 1.00 cm cuvette and was determined to be 0.818. calculate pka for hin.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
An acid-base indicator, hln, dissociates according to the following reaction in an aqueous solution....
Questions




Mathematics, 16.12.2021 19:30

Mathematics, 16.12.2021 19:30



Mathematics, 16.12.2021 19:30

English, 16.12.2021 19:30

Advanced Placement (AP), 16.12.2021 19:30

Mathematics, 16.12.2021 19:30

Physics, 16.12.2021 19:30

Mathematics, 16.12.2021 19:30

Spanish, 16.12.2021 19:30

History, 16.12.2021 19:30





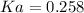
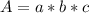
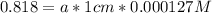
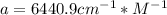
![x_{HIn]+x_{In}=1](/tpl/images/0397/0230/d5361.png)
![x_{HIn]=1-x_{In}](/tpl/images/0397/0230/0ee73.png)
![a=x_{In}*20060cm^{-1}*M^{-1}+ x_{HIn]*2929cm^{-1}*M^{-1}](/tpl/images/0397/0230/9addd.png)
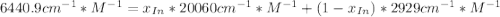
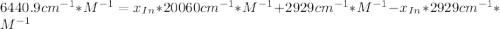
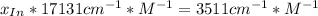
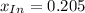
![x_{HIn]=1-0.205=0.795](/tpl/images/0397/0230/0ce5b.png)
![Ka=\frac{[In]}{[HIn]}](/tpl/images/0397/0230/065b2.png)