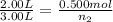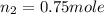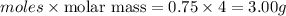Asteel container with a movable piston contains 2.00 g of helium which was held at a constant temperature of 25 °c. additional helium was pumped into the container and the piston adjusted so that the gas pressure remained constant. how many grams of helium were added to the cylinder if the volume was changed from 2.00 l to 3.00 l? a) 0.700 gb) 2.00 gc) 1.8 gd) 1.00 ge) 9.7 gf) 5.63 gg) 4.63 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2


Chemistry, 23.06.2019 16:10
Which quote from the passage best illustrates della’s optimism? “…and at length she was able to look up with dim eyes and a smile and say: "my hair grows so fast, jim! ” “…her heart had simply craved and yearned over them without the least hope of possession.” “and now, they were hers, but the tresses that should have adorned the coveted adornments were gone.” “beautiful combs, pure tortoise shell, with jewelled rims--just the shade to wear in the beautiful vanished hair.”d, ardent, most likely means? gloomy calm passionate innocent
Answers: 2
You know the right answer?
Asteel container with a movable piston contains 2.00 g of helium which was held at a constant temper...
Questions





History, 21.10.2021 14:00

History, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00


Engineering, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00


Physics, 21.10.2021 14:00

Physics, 21.10.2021 14:00


Biology, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00

Computers and Technology, 21.10.2021 14:00



Mathematics, 21.10.2021 14:00

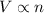
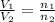
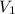 = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L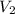 = final volume of gas = 3.00 L
= final volume of gas = 3.00 L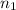 = initial moles of gas =
= initial moles of gas =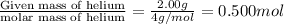
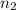 = final moles of gas = ?
= final moles of gas = ?