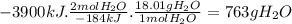Chemistry, 30.11.2019 05:31 HistoryLee
According to the following thermochemical equation, what mass of h2o (in g) must form in order to produce 3900 kj of energy?
sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj 216 g 382 g 763 g 408 g 272 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
According to the following thermochemical equation, what mass of h2o (in g) must form in order to pr...
Questions

Mathematics, 19.08.2020 14:01

French, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01



Medicine, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01


Computers and Technology, 19.08.2020 14:01


Mathematics, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01

English, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01



Mathematics, 19.08.2020 14:01


Health, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01