Chemistry, 30.11.2019 04:31 endyrocks19
Solid aluminum hydroxide reacts with a solution of hydrobromic acid. write a balanced molecular equation and a balanced net ionic equation

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

You know the right answer?
Solid aluminum hydroxide reacts with a solution of hydrobromic acid. write a balanced molecular equa...
Questions


Social Studies, 17.03.2021 23:40



Chemistry, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

English, 17.03.2021 23:40


English, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Arts, 17.03.2021 23:40

Physics, 17.03.2021 23:40

Computers and Technology, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

History, 17.03.2021 23:40

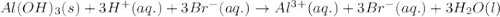
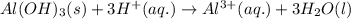
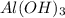 ) is a base and hydrobromic acid (HBr) is a strong acid.
) is a base and hydrobromic acid (HBr) is a strong acid.