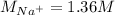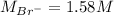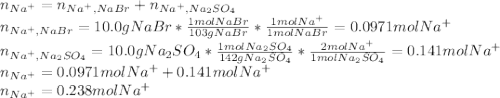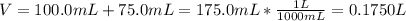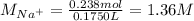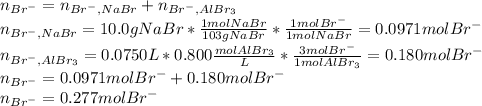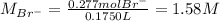Chemistry, 30.11.2019 03:31 caplode7497
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml solution. this solution is then mixed with 75.0 ml of a 0.800 m aqueous solution of albr3. calculate the concentration (m) of na+ and br− in the final solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml...
Questions

Mathematics, 20.05.2021 18:20





Mathematics, 20.05.2021 18:20

English, 20.05.2021 18:20

Mathematics, 20.05.2021 18:20


Mathematics, 20.05.2021 18:20

English, 20.05.2021 18:20


French, 20.05.2021 18:20


Mathematics, 20.05.2021 18:20

History, 20.05.2021 18:20

Biology, 20.05.2021 18:20


History, 20.05.2021 18:20

Mathematics, 20.05.2021 18:20