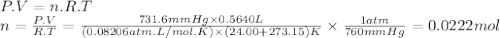Chemistry, 30.11.2019 03:31 fattypickeltoefungus
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experiment, a sample of kclo3 reacts and the gas produced is collected by water displacement. the gas sample has a temperature of 24.00 °c, a volume of 564.0 ml, and a pressure of 754.0 mm hg.
calculate the amount (in moles) of oxygen gas produced in the reaction. the vapor pressure of water is 22.38 mm hg at 24.00 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experime...
Questions

Mathematics, 14.07.2019 07:00


History, 14.07.2019 07:00






History, 14.07.2019 07:00


Mathematics, 14.07.2019 07:00




Social Studies, 14.07.2019 07:00