
Chemistry, 30.11.2019 03:31 georgesarkes12
Enter a balanced equation for the reaction between aqueous lead(ii) nitrite and aqueous potassium bromide to form solid lead(ii) bromide and aqueous potassium nitrite. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Enter a balanced equation for the reaction between aqueous lead(ii) nitrite and aqueous potassium br...
Questions

Mathematics, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00

Computers and Technology, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00

History, 23.02.2021 01:00


Social Studies, 23.02.2021 01:00




Mathematics, 23.02.2021 01:00

Mathematics, 23.02.2021 01:00

Mathematics, 23.02.2021 01:00


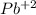 since oxidation number of lead is 2 as indicated lead (II)nitrite. The number in parentheses indicates the oxidation number.
since oxidation number of lead is 2 as indicated lead (II)nitrite. The number in parentheses indicates the oxidation number.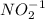 bears the charge -1.
bears the charge -1. 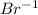 , it is a halogen and bears the charge -1.
, it is a halogen and bears the charge -1.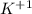 , it is an alkali metal.
, it is an alkali metal.

