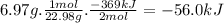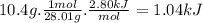The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium hydroxide(aq) and hydrogen(g). 2na(s) + 2h2o(l)2naoh(aq) + h2(g) h = -369 kj when 6.97 grams of sodium(s) react with excess water(l), kj of energy are .
2) the following thermochemical equation is for the reaction of carbon monoxide(g) with water(l) to form carbon dioxide(g) and hydrogen(g).
co(g) + h2o(l)arrow. gif co2(g) + h2(g) delta16-1.gifh = 2.80 kj
when 10.4 grams of carbon monoxide(g) react with excess water(l), kj of energy are .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
You know the right answer?
The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium...
Questions

Social Studies, 26.04.2020 10:46

Mathematics, 26.04.2020 10:46

English, 26.04.2020 10:46


Mathematics, 26.04.2020 10:46

English, 26.04.2020 10:46


Mathematics, 26.04.2020 10:47

English, 26.04.2020 10:47


Chemistry, 26.04.2020 11:06

Social Studies, 26.04.2020 11:06


Mathematics, 26.04.2020 11:06





Chemistry, 26.04.2020 11:07