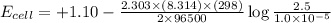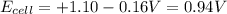The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this reaction is v when the concentration of [cu2+]=1.0⋅10−5m and [zn2+]=2.5m. zn (s) + cu2+ (aq) → cu (s) + zn2+ (aq) the standard cell potential () for the reaction below is . the cell potential for this reaction is when the concentration of and (s) + (aq) (s) + (aq) 0.78 1.10 0.94 1.26 1.42

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this r...
Questions


Mathematics, 07.10.2019 13:50

Mathematics, 07.10.2019 13:50






Chemistry, 07.10.2019 13:50



World Languages, 07.10.2019 13:50

Mathematics, 07.10.2019 13:50


History, 07.10.2019 13:50


Social Studies, 07.10.2019 13:50

Mathematics, 07.10.2019 13:50


Biology, 07.10.2019 13:50

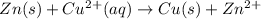
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Cu^{2+}]}](/tpl/images/0396/8274/2e6f5.png)
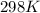
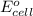 = standard electrode potential of the cell = +1.10 V
= standard electrode potential of the cell = +1.10 V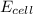 = emf of the cell = ?
= emf of the cell = ?