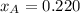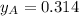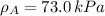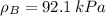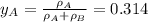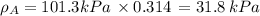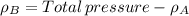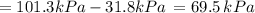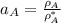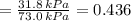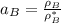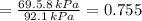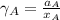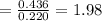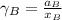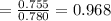By measuring the equilibrium between liquid and vapour phases of a solution at 30°c at 1.00 atm, it was found that xa = 0.220 when ya = 0.314. calculate the activities and activity coefficients of both components in this solution on the raoult’s law basis. the vapour pressures of the pure components at this temperature are: pa = 73.0 kpa and pb = 92.1 kpa. (xa is the mole fraction in the liquid and ya the mole fraction in the vapour.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
By measuring the equilibrium between liquid and vapour phases of a solution at 30°c at 1.00 atm, it...
Questions



Social Studies, 03.03.2020 20:03


English, 03.03.2020 20:03






English, 03.03.2020 20:03




Geography, 03.03.2020 20:04

History, 03.03.2020 20:04


Mathematics, 03.03.2020 20:04


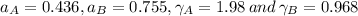 .
.