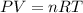Chemistry, 30.11.2019 02:31 megamegs80
Calculate the volume of the gas when the pressure of the gas is 1.60 atm at a temperature of 298 k. there are 160. mol of gas in the cylinder. the value for the universal gas constant r is 0.08206 l⋅atm/(mol⋅k) .
express your answer numerically to four significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Calculate the volume of the gas when the pressure of the gas is 1.60 atm at a temperature of 298 k....
Questions


Mathematics, 13.04.2020 17:50

English, 13.04.2020 17:50


History, 13.04.2020 17:50

Mathematics, 13.04.2020 17:50

English, 13.04.2020 17:50

Business, 13.04.2020 17:50

Chemistry, 13.04.2020 17:50


Mathematics, 13.04.2020 17:50

English, 13.04.2020 17:50


Mathematics, 13.04.2020 17:50

History, 13.04.2020 17:50

Mathematics, 13.04.2020 17:50


History, 13.04.2020 17:50