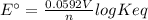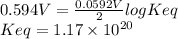Chemistry, 30.11.2019 02:31 mhuerta71001
Interactive activity—the relationship among e°cell, keq, and gibbs free energy in an electrochemical cell, the potential difference between two electrodes under standard conditions is known as the standard cell potential (e∘cell). the standard cell potential can be used to identify the overall tendency of a redox reaction to occur spontaneously. the spontaneity of a reaction is identified using the gibbs free energy δg∘. δg∘ is related to e∘cell. e∘cell and δg∘ are also related to equilibrium constant keq of the reaction. select the image to explore the activity that shows how e∘cell, keq, and δg∘ are related to each other. launch activity in the activity, you should see a triangle, whose three vertices represent keq, e∘cell, and δg∘. you can select two vertices and determine the relation between them. you can then reset the activity and select the next two quantities. constants the following values may be useful when solving this tutorial. constant value e∘cu 0.337 v e∘ni -0.257 v r 8.314 j⋅mol−1⋅k−1 f 96,485 c/mol t 298 k part a in the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are cu2+(aq)+2e−→cu(s) and ni(s)→ni2+(aq)+2e− the net reaction is cu2+(aq)+ni(s)→cu(s)+ni2+(aq) use the given standard reduction potentials in your calculation as appropriate. express your answer numerically to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

You know the right answer?
Interactive activity—the relationship among e°cell, keq, and gibbs free energy in an electrochemical...
Questions



Mathematics, 02.07.2021 21:20



English, 02.07.2021 21:20

Business, 02.07.2021 21:20



Computers and Technology, 02.07.2021 21:20




Social Studies, 02.07.2021 21:20




Chemistry, 02.07.2021 21:20