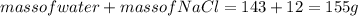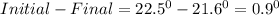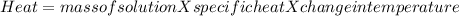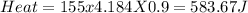1. a calorimeter contains 143 g of water at 22.5°c. a 12 g sample of nacl is added to the water in the calorimeter. after the solid has dissolved, the temperature of the water is 21.6°c. calculate the enthalpy of solution for dissolving sodium chloride. assume that no heat is lost to the calorimeter or the surroundings and that the specific heat of the solution is the same as that of pure water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3


Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
1. a calorimeter contains 143 g of water at 22.5°c. a 12 g sample of nacl is added to the water in t...
Questions

Mathematics, 25.10.2019 17:43

Biology, 25.10.2019 17:43






Social Studies, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

History, 25.10.2019 17:43





Arts, 25.10.2019 17:43


Chemistry, 25.10.2019 17:43


English, 25.10.2019 17:43