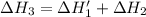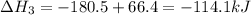Chemistry, 30.11.2019 01:31 tstaples02
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to create nitrogen dioxide. 2no (g) + o2 (g) → 2no2 (g)
calculate the standard reaction enthalpy for the above reaction using the following thermodynamic data.
n2 (g) + o2 (g) → 2no (g) ∆h˚1 = 180.5 kj
n2 (g) + 2o2 (g) → 2no2 (g) ∆h˚2 = 66.4 kj
-252.4 kj/mol rxn
-114.1 kj/mol rxn
-100.3 kj/mol rxn
-246.9 kj/mol rxn

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1


Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to cr...
Questions

Mathematics, 09.03.2021 01:50

Chemistry, 09.03.2021 01:50



Chemistry, 09.03.2021 01:50


Mathematics, 09.03.2021 01:50

Chemistry, 09.03.2021 01:50

Mathematics, 09.03.2021 01:50

Mathematics, 09.03.2021 01:50

Mathematics, 09.03.2021 01:50





Biology, 09.03.2021 01:50

History, 09.03.2021 01:50


Biology, 09.03.2021 01:50

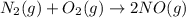 (1)
(1) 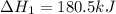
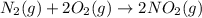 (2)
(2) 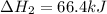
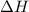 for the following reaction i.e,
for the following reaction i.e, (3)
(3) 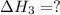
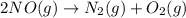 (1')
(1') 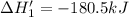
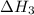 for the reaction will be:
for the reaction will be: