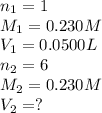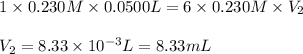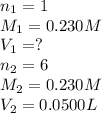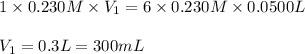For the following reaction between mohr's salt (iron as feso_4(nh_4)_2so_4 6h_2o) and potassium dichromate (dichromate as k_2cr_2o_7), determine the volume (in milliliters) of a 0.230 m solution of mohr's salt that is needed to fully react with 0.0500 l of 0.230 m potassium dichromate. (the reaction is shown in its ionic form in the presence of a strong acid.)
cr_2o_7^2- + 6fe^2+ + 14h^+ rightarrow 2cr^3+ + 6fe^3+ + 7h_2o
mohr's salt volume = ml
for the same reaction, what volume (in milliliters) of 0.230 m potassium dichromate is required to fully react with 0.0500 l of a 0.230 m solution of mohr's salt
potassium dichromate volume = ml

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
For the following reaction between mohr's salt (iron as feso_4(nh_4)_2so_4 6h_2o) and potassium dich...
Questions




History, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01

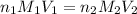
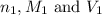 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of 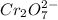
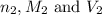 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of ![Fe^{2+]](/tpl/images/0396/5656/ad855.png)