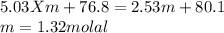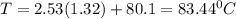Chemistry, 30.11.2019 01:31 mariakelley15
Imagine two solutions with the same concentration and the same boiling point, but one has benzene as the solvent and the other has carbon tetrachloride as the solvent. determine that molal concentration, m (or b), and boiling point, tb.
benzene boiling point=80.1 kb=2.53
carbon tetrachloride boiling point=76.8 kb=5.03

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1


Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
Imagine two solutions with the same concentration and the same boiling point, but one has benzene as...
Questions


Physics, 13.05.2021 14:00







Mathematics, 13.05.2021 14:00

Health, 13.05.2021 14:00

Mathematics, 13.05.2021 14:00

Biology, 13.05.2021 14:00

Mathematics, 13.05.2021 14:00


Chemistry, 13.05.2021 14:00


English, 13.05.2021 14:00

Chemistry, 13.05.2021 14:00

Social Studies, 13.05.2021 14:00

English, 13.05.2021 14:00

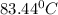
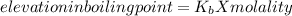
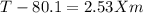
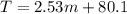 ....(1)
....(1)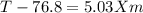
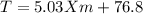 ...(2)
...(2)