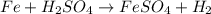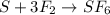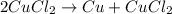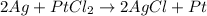Chemistry, 30.11.2019 01:31 preciosakassidy
Identify the following redox reactions by type. check all that apply. (a) fe + h2so4 → feso4 + h2 combination decomposition displacement disproportionation (b) s + 3f2 → sf6 combination decomposition displacement disproportionation (c) 2cucl → cu + cucl2 combination decomposition displacement disproportionation (d) 2ag + ptcl2 → 2agcl + pt combination decomposition displacement disproportionation

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

You know the right answer?
Identify the following redox reactions by type. check all that apply. (a) fe + h2so4 → feso4 + h2 co...
Questions



Social Studies, 08.11.2019 21:31









Spanish, 08.11.2019 21:31





History, 08.11.2019 21:31

English, 08.11.2019 21:31

Mathematics, 08.11.2019 21:31