Chemistry, 30.11.2019 00:31 jhaveen8335
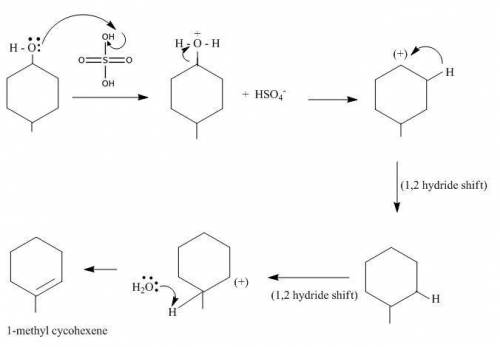
While the reaction in the lab text gives the product 4-methylcyclohexene, it is possible that several products are formed in this reaction, including 1-methylcyclohexene, because of 1,2-hydride shifts in the carbo-cation intermediate. show a step-wise, arrow-pushing mechanism for the formation of 1-methylcyclohexene.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
While the reaction in the lab text gives the product 4-methylcyclohexene, it is possible that severa...
Questions



Chemistry, 20.10.2019 19:30



History, 20.10.2019 19:30

Arts, 20.10.2019 19:30

Mathematics, 20.10.2019 19:30







Computers and Technology, 20.10.2019 19:30



History, 20.10.2019 19:30

English, 20.10.2019 19:30