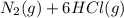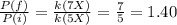Chemistry, 30.11.2019 00:31 dontworry48
Ammonia and chlorine react to form nitrogen and hydrochloric acid. if you react 2.0 l of ammonia with 3.0 l of chlorine at constant temperature in a fixed volume container, what is the ratio of the final and initial pressures (pria/pinta? 2nh3(g) + 3cl2 (g) → nz (g) + 6hci (8)
a. 0.71
b. 1.00
c. 1.40 d. 1.50
d. none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Ammonia and chlorine react to form nitrogen and hydrochloric acid. if you react 2.0 l of ammonia wit...
Questions



Mathematics, 05.02.2021 21:30

Mathematics, 05.02.2021 21:30








Mathematics, 05.02.2021 21:30




History, 05.02.2021 21:30

Arts, 05.02.2021 21:30


Computers and Technology, 05.02.2021 21:30

Computers and Technology, 05.02.2021 21:30

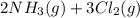 →
→