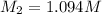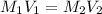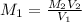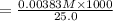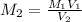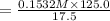Chemistry, 29.11.2019 06:31 sarahjdeering
A17.55 ml solution of potassium nitrate (kno3) was diluted to 125.0 ml, and 25.00 ml of this solution was then diluted to 1.000 × 103 ml. the concentration of the final solution is 0.00383 m. calculate the concentration of the original solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

You know the right answer?
A17.55 ml solution of potassium nitrate (kno3) was diluted to 125.0 ml, and 25.00 ml of this solutio...
Questions

Biology, 05.07.2019 07:10

Mathematics, 05.07.2019 07:10





Mathematics, 05.07.2019 07:10

Biology, 05.07.2019 07:10

English, 05.07.2019 07:10

English, 05.07.2019 07:10


Geography, 05.07.2019 07:10





Mathematics, 05.07.2019 07:10

History, 05.07.2019 07:10

English, 05.07.2019 07:10